Lipid oxidation in food
Lipid oxidation is one of the major sources for deterioration of food and food products. Rancidity is the phenomena related to reactions with molecular oxygen leading to volatile off-flavors.
It has been long recognized as a massive problem in the storage of fatty acids in foods [1-4].
Mechanisms of lipid oxidation
There are several mechanisms such as generation of reactive oxygen precursors and free radicals and affects many interactions of food constituents, leading to both desirable and undesirable products. Food lipids are very sensitive to oxidation, which can occur during manufacturing, storage, distribution and final preparation of foods. There are many catalytic systems that can oxidize lipids. Among these are light, temperature, enzymes, metals, metalloproteins and microorganisms. Most of these reactions involve some type of free radical and/or oxygen species.
In the last 50 years, lipid peroxidation has been the subject of extensive studies from the viewpoints of mechanisms, dynamics, product analysis, involvement in diseases, inhibition, and biological signaling. Lipids are oxidized by three distinct mechanisms; enzymatic oxidation, non-enzymatic, free radical-mediated oxidation, and non-enzymatic, non-radical oxidation. Each oxidation mechanism yields specific products and is influenced by several factors [5].
Factors that can influence the oxidation process:
- Temperature
- Light
- Oxygen
- Moisture
- Catalysts
- Ionizing Radiation
The pathways of lipid oxidation:
- Photooxidation
- Enzymatic oxidation
Lipid Oxidation and Off-Flavors [6]
The oxidation of unsaturated fatty acids is a complex phenomenon, which occurs in the presence of oxygen, non-enzymatically or enzymatically. The main mechanism of lipid oxidation in food is autoxidation, which consists of the reaction of triplet oxygen with organic compounds. Lipid autoxidation is a chain reaction process induced by free radicals described by three steps of initiation, propagation, and termination. Through the propagation stage, primary products of oxidation, namely lipid hydroperoxides, are produced.
The stability of the fatty acids mainly depends on the number of double bonds as shown in Table 2.
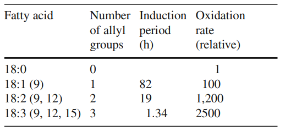
These products are unstable and break down to a number of secondary oxidation products such as ketones, aldehydes, hydrocarbons, alcohols, and volatile organic acids among others; some of which have undesirable odors with extremely low threshold values as shown in the table below.
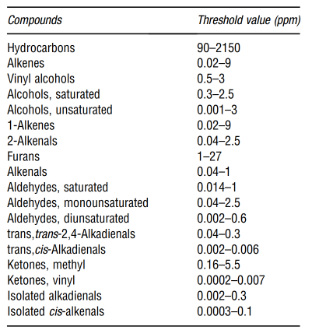
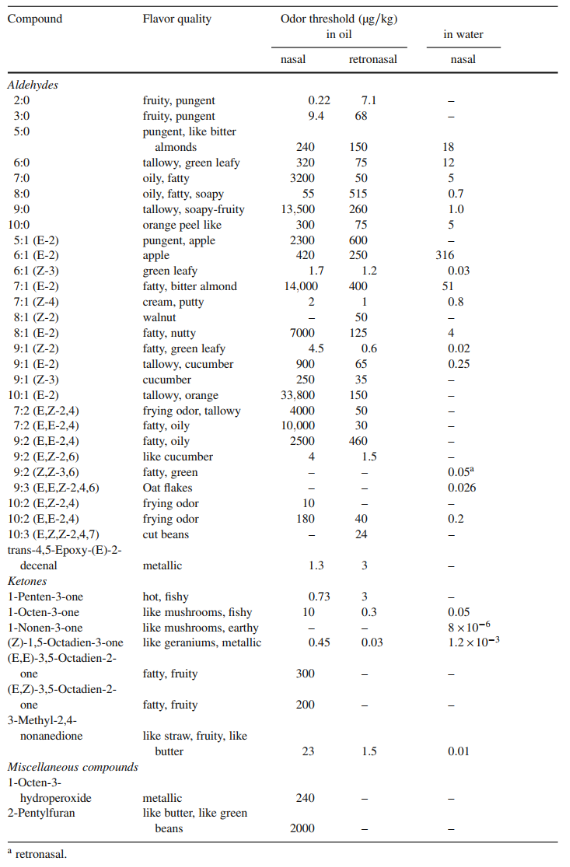
A more detailed information about the sensory properties and their thresholds can be found in Table 3.
References
- Lipid oxidation in food, an overview. J. R. Vercellotti et al., ACS Sympoisum Series 500, 1992, Volatile lipid oxidation products https://pubs.acs.org/doi/book/10.1021/bk-1992-0500
- Oxidation of lipids in foods. M. Ahmed et al., Sarhad Journal of Agriculture 2016, 32(3): 230-238. http://dx.doi.org/10.17582/journal.sja/2016.32.3.230.238
- Food Chemistry. H.D. Belitz et al, Lipids 2009, 158-247, ISBN 978-3-540-69934-7
- Volatile lipid oxidation products. E. N. Frankel, Proc. Lipid Res, 1982, 22, 1-33
- Lipid peroxidation: Mechanisms, inhibition, and biological effects. E. Niki et al., Biochemical and Biophysical Research Communications 338 (2005) 668–676
- Lipid-Derived Flavours and Off-Flavours in Food. F. Shahidi, A. Abad, Encyclopedia of Food Chemistry, 2019, Vol 2, 182-192 https://doi.org/10.1016/B978-0-08-100596-5.21666-1

